Abstract
Heart failure with preserved ejection fraction (HFpEF) is characterized by symptoms of HF with a left ventricular ejection fraction (LVEF)>50% and elevated cardiac filling pressures. HFpEF accounts for nearly half of HF and is associated with aging and systemic inflammation. The etiology of HFpEF is poorly understood, and there are few evidence-based therapies for HFpEF. Clonal hematopoiesis of indeterminate potential (CHIP) is characterized by cancer-associated driver mutations (at a VAF of >2%) in the blood cells of individuals without hematologic malignancies. CHIP is associated with atherosclerosis and heart failure with reduced ejection fraction (HFrEF), but there are no published studies linking CHIP and HFpEF.
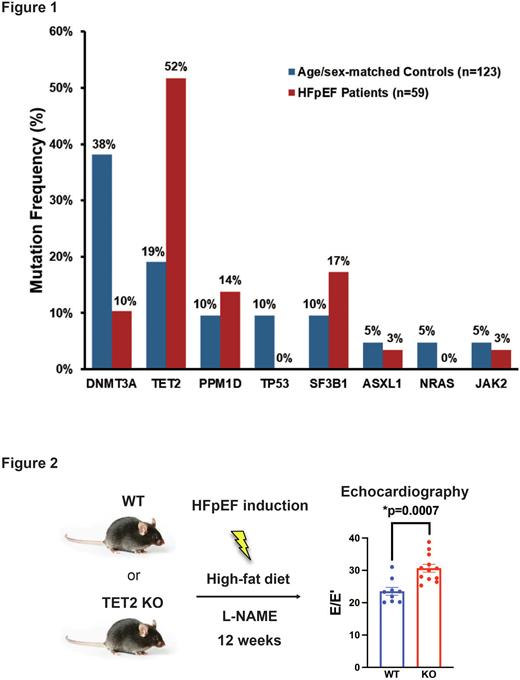
To assess the prevalence of CHIP in HFpEF, we accrued blood samples from 59 patients at UTSW with a validated diagnosis of HFpEF (mean age 72). To detect CHIP, we used a hybridization capture-based next-generation sequencing assay covering 24 genes known to cause CHIP. We identified 31 mutations (mean coverage 658x) to establish the presence of CHIP in 31.1% (n=19) of patients, as compared with 15.4% of age/gender matched controls (n=123) (Fig. 1). Nine patients (47.4%) had two mutations and two patients had three mutations (10.5%), and the mean VAF was 8.99%. The most frequent mutation was in TET2 (n=10, 52.6%), and notably 50% (n=5) of patients with TET2-mutated CHIP harbored two TET2 mutations. The second most frequent mutation was in SF3B1 (n=5, 26.3%). In contrast, mutations in DNMT3A, the most frequently mutated CHIP-driver gene in HFrEF patients and our age matched controls, were observed at a much lower rate (n=3, 15.8%). Given the role for ascorbate as a TET2 co-factor, we measured ascorbate in a subset of patients (n=18) using LC-MS/MS, finding a trend towards an association between low ascorbate levels and supranormal LVEF (Pearson r -0.41, p=0.12), which is associated with adverse cardiovascular outcomes. Since SF3B1 mutations may promote altered iron homeostasis, we evaluated ferritin levels, finding them to be higher (mean 291 ng/ml) in SF3B1-mutated CHIP patients as compared with patients with non-SF3B1-mutated CHIP (mean 112 ng/ml, p=0.048) and patients without CHIP (mean 103 ng/ml, p=0.0049).
Given the high prevalence of TET2 mutations, we sought to determine if TET2-mutated CHIP directly contributes to HFpEF pathogenesis. We used a well-established mouse model in which concurrent metabolic and hypertensive stress elicited by a high-fat diet (HFD) and inhibition of nitric oxide (NO) synthase by L-NAME successfully recapitulates the myriad clinical features of HFpEF (Nature 2019;568:351). We induced hematopoietic-specific deletion of TET2 by injecting Mx1-cre;Tet2fl/fl mice (TET2 KO, n=12) or Cre-negative Tet2fl/fl control mice (WT, n=9) with polyinosinic-polycytidylic acid. Six to eight weeks after Tet2 deletion, mice were placed on HFpEF treatment (HFD plus L-NAME) for 12 weeks. Despite similar systemic responses such as weight gain, hypertension, and glucose intolerance, TET2 KO mice developed exacerbated diastolic dysfunction as demonstrated by significantly elevated E/E’ in echocardiographic studies (30.3% increase, p=0.007) (Fig.2). These results suggest that loss of TET2 promotes HFpEF likely through direct effects on the heart. We obtained similar results when we induced HFpEF in WT mice transplanted with bone marrow (BM) from TET2 KO vs. WT donors, suggesting that TET2 loss promotes HFpEF via BM-derived immune cells infiltrating the heart, rather than pre-existing tissue resident immune cells. To test if loss of TET2 leads to molecular alterations in immune cells to promote HFpEF, we purified CD11b-positive cells from the hearts of TET2 KO mice and WT controls following 12 weeks of HFpEF induction and performed RNA-sequencing. In cells isolated from TET2 KO animals, we identified 2,008 DEGs (FDR <0.1), and gene-ontology analysis revealed significant enrichment (FDR<0.05) for genes associated with inflammation (NLRP3 inflammasome, IL-1b production) and fibrosis (TGF1b).
In sum, our data demonstrate a high prevalence of TET2-mutated CHIP in patients with HFpEF, and we confirm a direct effect of TET2-mutated blood cells on the development of HFpEF. We also identify SF3B1-mutated CHIP and disrupted iron homeostasis as a potential novel etiology for HFpEF. Together, these findings establish a rationale for targeting CHIP to treat or prevent HFpEF.
Disclosures
Bick:TenSixteen Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Kroger:Takeda: Consultancy, Honoraria; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding; DKMS: Research Funding; Riemser: Research Funding; Neovii: Honoraria, Research Funding; Amgen: Honoraria; Kite: Honoraria; Jazz: Honoraria; Sanofi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal